Novo Nordisk to acquire Inversago Pharma to develop new therapies for people living with obesity, diabetes and other serious metabolic diseases
Endocannabinoids interact with G-protein-coupled receptors (GPCRs), the same receptors that identify the psychoactive component of cannabis, resulting in a diverse array of effects across both the central nervous system and the peripheral regions. Signaling through GPCRs occurs via G-protein and β-arrestin-dependent pathways. The endocannabinoid system regulates metabolism, with CB1 receptor activation impacting energy balance and contributing to conditions like visceral obesity and metabolic syndrome (1,2). Rimonabant, the first selective CB1 receptor antagonist to be approved and used in the clinical practice, initially demonstrated highly promising in treating obesity-related issues was not approved by the FDA due to undesirable neuropsychiatric effects (3). Recently, MRI-1891, a peripheral CB1R antagonist, exhibits a strong bias towards inhibiting CB1R-triggered β-arrestin-2 (βArr2) recruitment compared to G-protein activation. Biased antagonism of CB1R signaling via βArr2 improves obesity-related insulin resistance without eliciting central nervous system-mediated adverse behavioral effects.
Liu et al. proved, that treatment with MRI-1891 leads to reduces food intake and body weight, without inducing anxiety even at high doses that partially occupy brain CB1Rs, in both obese wild-type and βArr2-knockout (KO) mice. Conversely, rimonabant elicited long-lasting hyperambulatory activity and caused strong anxiogenic responses in both strains, which indicates that altered βArr2 signaling is not involved in these behavioral responses to central CB1R blockade. In high-fat-diet-induced obesity/metabolic syndrome mouse model (DIO), MRI-1891 reduced food intake, body weight and hyperleptinemia in wild type and βArr2-KO mice, however improved obesity-related hyperinsulinemia and muscle insulin resistance in wild-type, but not in βArr2-KO mice. In C2C12 myoblasts, CB1R activation reduced insulin-induced akt-2 phosphorylation, which was prevented by MRI-1891, βArr2 knockdown, or CB1R-interacting protein overexpression. MRI-1891, unlike rimonabant, interacts with specific residues, enabling βArr2 bias, showing that CB1R induces muscle insulin resistance through βArr2 signaling, effectively alleviated by a selective CB1R antagonist with lowered potential for central nervous system side effects (4).
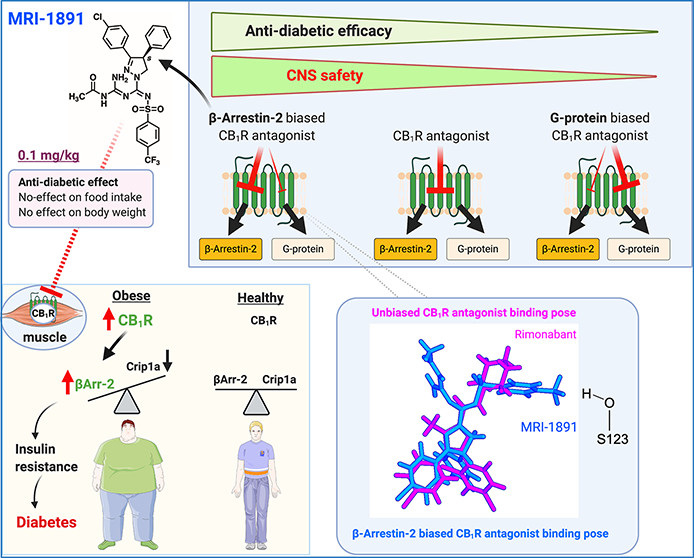
Figure from: Liu Z, Iyer MR, Godlewski G, Jourdan T, Liu J, Coffey NJ, Zawatsky CN, Puhl HL, Wess J, Meister J, Liow JS, Innis RB, Hassan SA, Lee YS, Kunos G, Cinar R. Functional Selectivity of a Biased Cannabinoid-1 Receptor (CB1R) Antagonist. ACS Pharmacol Transl Sci. 2021 Apr 8;4(3):1175-1187. doi: 10.1021/acsptsci.1c00048. PMID: 34151207; PMCID: PMC8204328.
The results described above are pioneering in the field of treating obesity and type II diabetes with cannabinoids, and form the basis for its commercial implementation. This research that initiated clinical trials of the drug INV-202 by Inversago Pharma. The results obtained so far are very promising, which is why Novo Nordisk has taken an interest in it. On August 10, 2023, it was announced that Novo Nordisk, will acquire Inversago Pharma for a potential $1.075 billion, contingent upon achieving specific developmental and commercial milestones. Inversago Pharma specializes in the development of CB1 receptor-based therapies targeting obesity, diabetes, and metabolic disorder complications. As part of the acquisition of Inversago, the company’s primary developmental asset INV-202, an oral CB1 inverse agonist, is included. INV-202 is engineered to selectively inhibit the receptor protein CB1, crucial for metabolism and appetite regulation, in peripheral tissues like adipose tissues, the gastrointestinal tract, kidneys, liver, pancreas, muscles, and lungs. INV-202 shows promise in reducing weight and currently undergoing a phase 2 trial for diabetic kidney disease. This collaboration is anticipated to enhance Novo Nordisk’s clinical development in obesity and related issues. Inversago Pharma’s CEO, François Ravenelle, sees the partnership unlocking the potential of CB1 blockers and expanding treatment options for metabolic syndrome. Inversago employs 22 individuals in Canada, who will work alongside Novo Nordisk to advance their technology. The acquisition is subject to regulatory approvals and is expected to conclude by the end of 2023.
Cannabinoid-based medications shows promising alternative for currently available treatment options. Since recently, the European Medicines Agency’s (EMA) safety committee, is assessing the potential risk of suicidal thoughts and self-harm associated with glucagon-like peptide-1 receptor (GLP-1) agonist medications, including Ozempic, Saxenda, and Wegovy. These drugs are used for weight loss and type 2 diabetes treatment. The review was initiated due to reports of such thoughts and behaviors linked to liraglutide and semaglutide usage. Authorities are analyzing about 150 cases, but it’s unclear if these issues are directly linked to the drugs or underlying conditions. The review, part of a signal procedure, is set to conclude in November 2023, including other GLP-1 receptor agonists. Despite wide usage, suicidal behavior isn’t currently listed as a side effect. Patients and professionals are advised to adhere to approved guidelines and report any side effects.
1. Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev [Internet]. 2006 [cited 2023 Aug 11];58(3):389–462. Available from: https://pubmed.ncbi.nlm.nih.gov/16968947/
2. Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab [Internet]. 2013 Apr 2 [cited 2023 Aug 11];17(4):475–90. Available from: https://pubmed.ncbi.nlm.nih.gov/23562074/
3. Le Foll B, Gorelick DA, Goldberg SR. The future of endocannabinoid-oriented clinical research after CB1 antagonists. Psychopharmacology (Berl) [Internet]. 2009 Jul [cited 2023 Aug 11];205(1):171–4. Available from: https://pubmed.ncbi.nlm.nih.gov/19300982/
4. Liu Z, Iyer MR, Godlewski G, Jourdan T, Liu J, Coffey NJ, et al. Functional Selectivity of a Biased Cannabinoid-1 Receptor (CB1R) Antagonist. ACS Pharmacol Transl Sci [Internet]. 2021 Jun 11 [cited 2023 Aug 11];4(3):1175–87. Available from: https://pubs.acs.org/doi/full/10.1021/acsptsci.1c00048